Electronics depend on chemistry more than you can imagine. To generate electricity, there are many methods used; one of them is chemical. Remember batteries, where electricity is generated because of chemical reactions inside the cell. In conductors like liquids and gases, electricity is carried not by electrons in solid constructions(copper, aluminum, etc.) but by ions – molecules with electric charges. Even non-distilled water contains enough ions to be conductive. Let’s go through several chemical effects of electricity.
Electrolysis
Electrolysis decomposes the liquid compound by passing an electric current through a liquid called an electrolyte(saltwater, copper sulfate, sulphuric acid). Electrolysis is used very widely in the industry, like electroplating metals, refining copper, and extraction of aluminum from ore. To make electrolysis happen, two conductors used cathode(-) and anode(-).
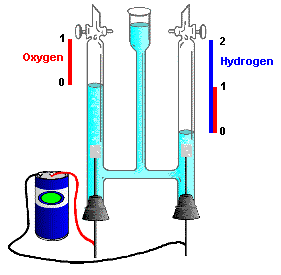
Electrolysis is one of the simplest ways to produce hydrogen, but this process requires too much electrical power that makes this process inefficient (about 40%) to be used in the industry. The alternative would be to use regenerative electrical plants(solar, wind, geothermal) to accumulate hydrogen.
Electroplating
It is the same electrolysis, jus there is used special electrolyte like copper sulfate. For instance, this enables tin-plating of steel, and depending on electrodes and anodes; there can be various plating alternatives like silver-plating and chromium-plating. Material to be plated should be connected as a cathode, while anode usually loses material.
Chemical cell
According to this principle, all batteries are generating electric power. Chemical cells convert chemical energy into electrical. If two different conductors(e.g., Copper and zinc) are placed in an electrolyte, an electric current starts flowing between electrodes.

Each different pair of electrodes generate a different potential. As long as there is an external circuit, electrons can flow through it from one electrode to another. Because zinc tends to lose electrons more readily than copper, zinc atoms in the zinc electrode lose electrons to produce zinc ions. Well, if you want to learn the electrolysis basics, the main difference between atoms and molecules, and further types of atoms, then visit https://askanydifference.com/difference-between-atom-and-molecule/. The net result is that zinc metal reacts with copper ions to produce zinc ions and copper metal.
Corrosion
Corrosion is a gradual destruction of metal in an environment where are conditions for simple cell reactions. Also, there are two electrodes required for corrosion. So if their metals are in contact with each other and there is the presence of electrolytes – corrosion will occur. To prevent corrosion, there are coating used like iron is coated by zinc. Or grease paint, plastic is used. In many cases, cathode protection is used where a noble metal is connected to a less noble and potential applied.
Non-rechargeable Leclanché cells
These cells generate about 1.5V of EMS, but if falls in continuous use due to polarisation of the electrode. These cells are better for us for intermittent purposes like torches, bells, indicators, etc. These cells are cheap and have a shelf life of about 2 years.

There are more dry cell technologies that enable to remain EMF constant for longer times. Ones are Mercury cells. These cells provide 1.3V for a longer time than Leclanché cells.
Rechargeable cells
Rechargeable cells are mo commonly used today as the main factor – the price is becoming acceptable for everyone. And there is another practical approach – these cells can be recharged after use. In other words, the conversion of chemical energy to electrical is reversible. There are many types of rechargeable cell technologies. One of the common is lead-acid and alkaline cells. These cells are usually used in cars. Bout other Rechargeable batteries, I would suggest reading in Wikipedia.






in the line above the first digram the anodes is +veil charged not negatively.otherwise ur website i liked one of the best.
I LIKE THIS ARTICLE VERY MUCH. BUT THERE MUST BE MORE CIRCUIT DIAGRAMS. ALSO A CIRCUIT DIAGRAM OF RECHARGEABLE CELL. AND HOW IT WORKS.
THANK YOU.
i hv learn alot in ths website.but i did’nt see the internal resistace of th cell & its’ problems.thankz alot & keep up.